Key Differences – Valency vs Valence Electrons
- For example, although elemental sodium is a metal, solid sodium chloride is an insulator, because the valence electron of sodium is transferred to chlorine to form an ionic bond, and thus that electron cannot be moved easily.
- Of valence electrons must be counted. This can be determined by using a periodic table. The group number matches the number of valence electrons. Example: Carbon is element 6. It is found in Group IV so has four valence electrons. Oxygen is element 8. It is found in Group VI so has six valence electrons.
- Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴.
Many valence electrons the element has 4. Draw the dots around the chemical symbol starting at the top and moving clockwise around the symbol.
Valency electrons and valence electrons are inter-related terms, and the key difference between valency and valence electrons is best explained in their definitions; valence electronsare the electrons in the outermost shell of an element whereas valency electronsare the number of electrons that should be accepted or removed to attain the nearest noble gas configuration. It is the electrons in the outermost shell that usually contribute to form chemical bonds. In some atoms, the number of valence electrons is equal to the number of valency electrons.

What are Valence Electrons?
The number of electrons in the outermost shell of an atom is called “valence electrons”. Because of this reason, the outermost shell of an atom is called “valence shell”. Most of the time, these are the electrons, which take part in chemical bonding. When elements form cations, they remove electrons from the valence shell. The number of valence electrons in an element determines the group in the periodic table.
What are Valency Electrons?
The number of electrons required to gain or lose to fill the outermost shell of an atom is called “valency electrons”. For a particular atom, the number of valency electrons depends on the number of valence electron in the atom. For Sodium, valency is equal to 1, because it removes the last electron in the outermost shell to achieve octet structure of the nearest noble gas.
|
What is the difference between Valency and Valence Electrons?
How To Calculate Valence Electrons
Definition of Valency and Valence Electrons
Valence Electrons: The electrons in the outermost shell of an atom are called “valence electrons”. For “s” and “p” group elements, the number of valence electrons is equal to their group number.
Example
Valency Electrons: The number of electrons that should be accepted or removed to attain the electron configuration of the nearest noble gas is called “valency electrons” or the “valence” of an atom.
In general, for metal elements (elements in group I, II and III), the number of valence electrons is equal to the number of valency electrons; they remove the electrons in the valence shell to achieve the octet structure.
But, non-metal elements accept electrons to achieve the electron configuration of the nearest noble gas. Therefore, the valency of non-metal elements is calculated by subtracting the total valence electrons from 8.
For Chlorine, Number of valency electrons = 8-7 =1
Characteristics of Valency and Valence Electrons
Valency and Valence Electrons of group VIII elements
Valence Electrons Example Problems
Valence Electrons: Group VIII elements are the noble gases, and they are chemically stable. Their outer shell is complete, and it contains eight electrons in the outermost shell (except Helium –He); so that group VIII electrons have eight valence electrons.
Valence Electrons Definition Chemistry Examples
Valency Electrons: Valency is a measure of the ability to form bonds with other elements or molecules. Noble gases do not accept or remove electrons to achieve the octet rule since they have already completed the last shell. Therefore, the valency of group VII elements equal to zero.
Image Courtesy:
“Electron shell 010 Neon – no label” by commons:User:Pumbaa (original work by commons:User:Greg Robson) – http://commons.wikimedia.org/wiki/Category:Electron_shell_diagrams (corresponding labeled version).(CC BY-SA 2.0 uk) via Commons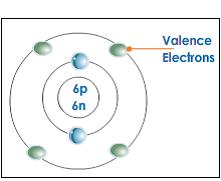
Valence Electrons Examples
Related posts:
